Reasons for Intraoperative Imaging
- Brain-shift-corrected navigation: OR navigation systems rely on preoperative imaging that doesn't consider changes in brain anatomy due to brain shift or deformations during surgery. By updating the information with intraoperative images the navigation and targeting is made more accurate.
- Monitoring/controlling thermal ablations: Temperature can be monitored during the procedure with MRI. Thus, radio-frequency and focused ultrasound thermal ablations can be controlled (formerly: MRI-guided interstitial laser brain surgery).
- Tumor control: Intraoperative imaging allows to verify the completeness of tumor resection at the end of surgery and, if necessary, removal of the residual. [1]
Image-Guided Neurosurgical Systems
One of the major challenges in neurosurgery is the localization of the tumor tissue and relevant anatomical structures within the brain during surgery. Therefore high accuracy is needed. Image-Guided Neurosurgical Systems (IGNS) help to take into account intraoperative deformations of the brain. Such systems register preoperative images to an intraoperative coordinate system of the patient.
Benefits of IGNS:
- possibility of fusion of anatomical and functional data
- accurate localization of lesions
- reduction of surgical time
- possibility of minimally invasive cranial openings
- decreased complication rates after surgery
A high rate of correlation between the preoperative and intraoperative data is necessary to ensure the precise localization of the tumor during surgery. This correlation is limited by the brain shift (intraoperative deformation of brain tissue). [2]
Compensation for brain shift
Intraoperative brain shift (deformation of brain tissue) during neurosurgeries is well-known. It is caused by gravity, tissue manipulation, tumor size, loss of CSF and medication. This effect greatly decreases the accuracy of image-guidance in neurosurgical procedures. Brain shift is a dynamic process and varies over time. So for image fusion of pre- and intra-operative data, the registration is not rigid. However, this assumption is still common.
Since intraoperative brain shift might be the most significant limitation of IGNS, the modeling of this phenomenon is crucial. To measure and quantify the magnitude and direction of brain shift, two approaches are commonly used:
- Direct measurement, compare preoperative MRI data with the data acquired directly on the brain surfaces of the patient during the surgery
- Analysis of registered pre- and intra-operative image data [2]
State-of-the-Art Intraoperative Imaging Modalities
Commercial IGNS assumes rigid registration, though brain shift is a nonrigid deformation and intraoperative navigation is based on preoperative images. Hence the accuracy of the surgical performance is affected. Without real time data the benefit of IGNS might turn into a risk. Which imaging modalities are used for intraoperative imaging of the brain and the clinical experience with them will be explained below. [2]
Intraoperative Fluorescence Imaging
Fluorescence-guided surgery is one of the rapidly emerging methods of surgical therapy and diagnostics. In some centers fluorescence-guided brain tumor surgery is routinely applied, in the others it is under active study in clinical trials.The most common used drugs for this purpose are fluorescein sodium, 5-aminolevulinic acid, and indocyanine green. But also less studied fluorescent stains, such as tetracyclines, cancer-selective alkylphosphocholine analogs, cresyl violet, acridine orange, and acriflavine, can be used for rapid tumor detection and pathological tissue examination.
Fluorescence Imaging is especially interesting for heterogeneous, complex tumor types, in which the extent of the tumor is not precisely identifiable, such as malignant glioma. [8]
figure: Open brain surgery in white light (left) and in blue light (right). [9]
Bibliography
1) Ferenc A.J. et.al. (2011) Intraoperative Imaging in Neurosurgery: Where Will the Future Take Us?, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4104677/
2) Siming Bayer et.al. (2017) Intraoperative Imaging Modalities and Compensation for Brain Shift in Tumor Resection Surgery, https://www.hindawi.com/journals/ijbi/2017/6028645/
3) Napolitano M. et.al. (2014) Glioblastoma surgery with and without intraoperative MRI at 3.0TGlioblastoma surgery with and without intraoperative MRI at 3.0T, http://ac.els-cdn.com/S0028377014000642/1-s2.0-S0028377014000642-main.pdf?_tid=d1a82cf0-4adf-11e7-b50c-00000aacb362&acdnat=1496771261_e11529c1588c2fd730597b68216a086e
4) Vitaz T.W. et.al. (2015) Techniques to Improve the Extent of Brain Tumor Resection — Awake Speech and Motor Mapping, and Intraoperative MRI, https://www.intechopen.com/books/molecular-considerations-and-evolving-surgical-management-issues-in-the-treatment-of-patients-with-a-brain-tumor/techniques-to-improve-the-extent-of-brain-tumor-resection-awake-speech-and-motor-mapping-and-intraop#F1
5) National center for image guided therapy, https://ncigt.org/brain-tumor-resection (access 05/06/17)
6) Bruzzone MG, D'Incerti L, Farina LL, Cuccarini V, Finocchiaro G., (2012) CT and MRI of brain tumors, https://www.ncbi.nlm.nih.gov/pubmed/22617235
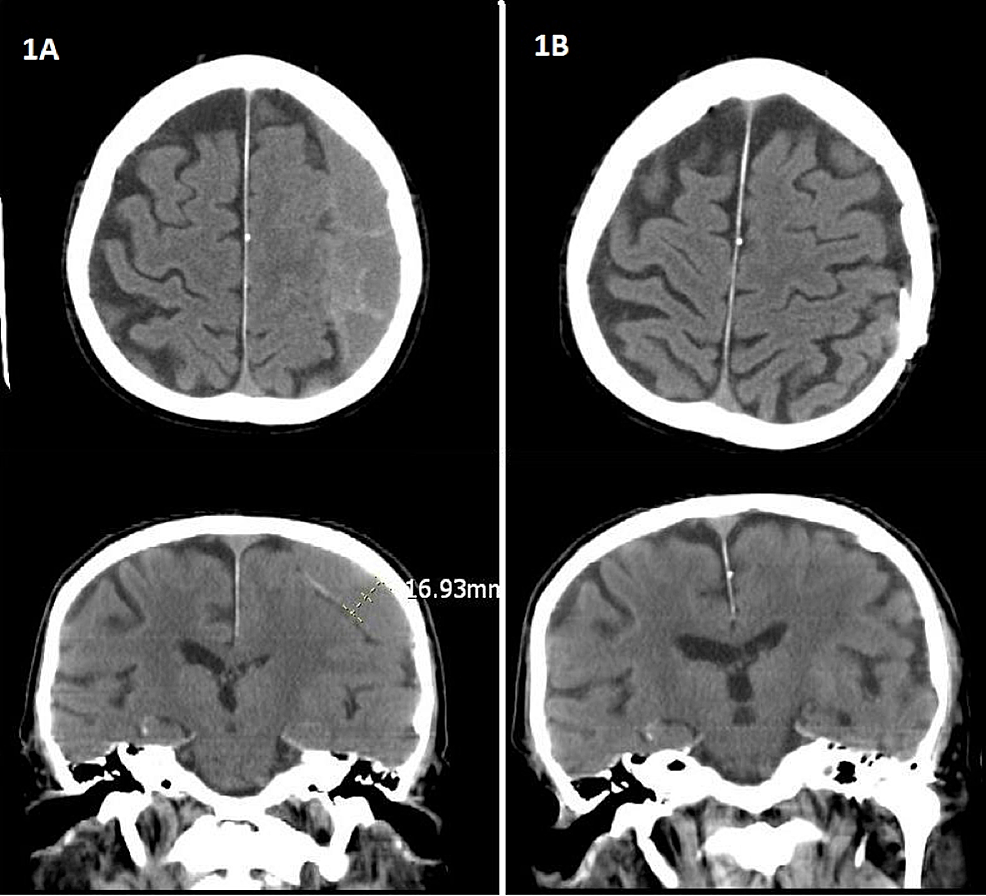
7) Figure 1: Brain CT Scan Images, http://www.cureus.com/articles/3113-is-subdural-peritoneal-shunt-placement-an-effective-tool-for-the-management-of-recurrentchronic-subdural-hematoma (access 06/06/17)
8) Belykh E. et.al. (2016) Intraoperative Fluorescence Imaging for Personalized Brain Tumor Resection: Current State and Future Directions
9) Gross Total Resection Rates in Contemporary Glioblastoma Surgery: Results of an Institutional Protocol Combining 5-Aminolevulinic Acid Intraoperative Fluorescence Imaging and Brain Mapping, http://www.neurosurgery-blog.com/archives/5077 (access 06/06/17)



2 Kommentare
Izabela Horvath sagt:
07. Juni 2017I liked the points and bullets approach with reasons and benefits, and that you give information about the first time the procedure was used:D As always, good text and tidy design.Good job!But I think you should also link the other 2 pages to the main page (now you only have the Augmented Reality)
Unbekannter Benutzer (ga38gis) sagt:
07. Juni 2017ah thanks, forgot about that