Introduction
This page will explore the medical aspects of the brain itself. We’ll start with brain anatomy, mainly focusing on large scale structures. Once we know the areas that make the brain up, we’ll cover the types of tumour that develop in each of these areas. Further down you’ll find information on the types of symptoms exhibited by patients with brain tumours, how they are diagnosed and how they are graded. But first, what is a brain tumour?
What is a brain tumour?
Both ‘brain tumour’ and ‘cancer’ are related terms but they refer to slightly different things. A brain tumour is a mass of cells in the brain caused by the uncontrolled growth of any of the types of cell that are found in the brain. The uncontrolled growth occurs when mutated cells, which arise naturally through errors in the cell reproduction process or through DNA damage by external agents, fail to automatically kill themselves (a process known as apoptosis).
This uncontrolled growth can be ‘graded’ based on how fast and aggressive the growth is. High grade brain tumours are fast growing, and are more likely to spread. The condition characterized by the presence of high grade tumours is known as ‘cancer’.
It is not known exactly what causes the uncontrolled growth that leads to brain tumours but we do know that certain genetic conditions are a risk factor as is radiation, in particularly having radiotherapy to the head as young child.
Figure 1: Uncontrolled Groth of Tumour Cells through Errors in Apoptosis (Source: Jake Burton).
Brain Anatomy
The brain consists of 3 main parts. The cerebrum, brainstem and cerebellum. Whilst each of these parts has its own specialised function, the overall function of the brain is to process sensory information and coordinate the response by the rest of the body. Whether that’s a visible action, like a muscle movement or an invisible action like the modulation of internal organ function (McDougall et al., 2015).
The brain is an important part of the human nervous system, itself divided into two parts, the central and the peripheral nervous systems. The central nervous system (CNS) is composed of the brain and spinal cord whilst the peripheral nervous system (PNS) consists of everything else. The PNS includes the autonomic nervous system, this is the system that regulates involuntary actions like breathing.
If you were to look at a human brain you would immediately see a number of key features, even before you cut it open. Some of the major landmarks are:
- Longitudinal fissure - a deep grove that seperates cerebral hemispheres at the bottom of which is the corpus callosum, a thick nerve bundle which connects together the two hemispheres.
- Gyri - these are the thick folds you can see on the surface of the brain.
- Suici - the shallow groves of the brain.
Cerebrum
The cerebrum is the largest part of the brain and is structured into two halves (hemispheres), a left hemisphere and a right hemisphere. These hemispheres are separated from one another by a groove known as the longitudinal fissure. As well as being separated left and right, the cerebrum is also composed differently from top to bottom. The outer layers of the two hemispheres consist of grey matter while the underlying area consists of white matter.
It’s not all Grey & White
To understand the difference between grey and white matter we need to know a little bit about Neurons – the workhorse cell of the human nervous system. Neuron’s might look complicated but consist of only 3 parts: a cell body, dendrites and an axon. The human brain contains 86,000,000,000 such cells, but we are by no means the animal with the most neurons, the African elephant brain has 257,000,000,000 neurons.
The role of the neuron is to carry electrochemical signals, these signals travel from the dendrites toward the cell body (also called the soma) and out along the axon where they pass to the dendrites of adjacent neurons. In some cases, the axon of the neuron is very long (relatively speaking) in which case the neuron will often be myelinated. Myelinated axons are surrounded by a fatty white substance called Myelin. Myelin is an insulating material and its increases the speed at which electrical impulses travel along the axon. Rather than moving like a wave along the axon, the electrical signal in a myelinated neuron propagates in a ‘jumping’ fashion via saltatory conduction. In Figure 3 the neuron is shown with a myeling sheath provided by Schwann cells. This is common in the peripheral nervous system and along some nerves near the brain. In the brain however the myelin is provided by a type of glial cell called an Oligodendrocyte.
Figure 2: Typical Neuron Structure (Cell Nucleus in Green) (Source: Jake Burton).
Figure 3: Components of a Myelinated Neuron (Source: Jake Burton).
Figure 4: Firing of an Unmyelinated Neuron Showing Direction of Signal Propagation (Neurotransmitter in Red) (Source: Jake Burton).
Figure 5: Firing of a Myelinated Neuron, Signal 'Jumps' through Myelinated Sections (Source: Jake Burton).
The presence or absence of myelin, which is white, gives rise to the distinction between white and grey matter. White matter consists mainly of bundles of myelinated axons whilst grey matter contains mostly cell bodies, dendrites and short unmyelinated axons. The grey matter regions of the brain are involved in muscle control and sensory perception (Miller et al., 1980). It is the purpose of the white matter to connect these various areas of grey matter together.
The outer layer of the cerebrum containing the grey matter is called the cerebral cortex. This area is often divided topographically into 4 lobes. These lobes are named after the bones of the skull which protect them. They are, from back to front: occipital lobe, temporal lobe, parietal lobe and frontal lobe.
Subcortical Structures
Physically below the cerebral cortex but still in the cerebrum are a number of subcortical structures: the hippocampus, basal ganglia and olfactory bulb. Just as humans have two sides to the cerebrum, we also have two hippocampi. These are located one in each side of the cerebrum. The hippocampus is involved in the consolidation of memory and in spatial memory which we use for navigation. The basal ganglia are a collection of neurons which are strongly connected to the cerebral cortex, thalamus and brainstem. It is responsible for a variety of functions such as the control of voluntary motor movements, procedural learning, emotion and habit formation. The olfactory bulb is a collection of neurons responsible for providing us with our sense of smell.
Other Forebrain Structures
The cerebrum is located in the ‘forebrain’, this is the front most part of the brain. This region, along with the midbrain and hindbrain are the tree primary parts of the brain formed during the development of the central nervous system. Also in the forebrain are the Thalamus and Hypothalamus.
The thalamus is a large mass of grey matter between the cerebral cortex and the midbrain. It is linked to the cerebral cortex in all directions by bundles of nerve fibres. Due to these connections, it is thought to act as a relay between different subcortical areas and the cerebral cortex. All the sensory systems except smell send signals to the cerebral cortex via the Thalamus. In addition to simply relaying the sensory information, it is also thought that the Thalamus also processes sensory information. The thalamus also plays a role in the regulation of sleep and wakefulness cycles. Damage to the thalamus can result in a coma.
The hypothalamus, a word literally meaning “under the thalamus”, is a small cluster of neurons which in humans is approximately the size of an almond. The hypothalamus is part of the limbic system, the regions of the brain involved in motivation, emotion, learning, and memory. The hypothalamus controls body temperature, hunger, thirst, tiredness and sleep patterns.
However, the primary role of the hypothalamus is to link the nervous and endocrine systems (the latter being the system or hormone secreting glands found throughout the body). This is done via a link to the pituitary gland which is located nearby. To control the pituitary gland the hypothalamus synthesizes and later releases a type of hormone called ‘releasing hormones’. These hormones are chemical signals that in turn stimulate or inhibit the release of hormones by the pituitary gland.
Hindbrain Structures
The cerebellum, pons and medulla oblongata are the structures of the hindbrain. They are physically located near the base of the brain, near the connection to the spinal cord. Together the pons and medulla oblongata form the brain stem. The brain stem controls our involuntary actions, like breathing and digestion.
Pons
The pons contains several neural pathways which link together the different parts of the brain. In particular the pons relay’s information from the brain down to the cerebellum and medulla oblongata as well as up in the other direction, up into the thalamus. Brain stem tumours found in children usually originate in the pons.
Medulla Oblongata
The medulla oblongata lies directly below the pons and is the part of the brain responsible for carrying messages from higher parts to the spinal cord further down. The spinal cord itself consists of all a bundle of nerve fibres which pass down out of the brain to different parts of the body. Like the brain, the spinal cord is protected by a surrounding of CSF.
In additional to this role the medulla oblongata is also part of the autonomic nervous system and is responsible for the regulation of some involuntary functions like heart rate and breathing as well as the gagging, sneezing, swallowing and coughing reflexes.
Cerebellum
The cerebellum, sometimes called the hindbrain itself despite being a component thereof, is the second largest structure in the brain. It is located adjacent to the pons, and medulla oblongata and extends slightly further down at the very back of the skull beneath the cerebral hemispheres. It is sometimes also called the “little brain” because anatomically it has the appearance of being an entirely separate structure to the brain.
The cerebellum is involved in motor control functions, that is, the coordination of our limbs and muscle movements and therefore the cerebellum affects both our balance and coordination. Though it does not actually initiate movement itself, the cerebellum integrates sensory information from the body in order to fine-tune muscle control.
There is some research that suggests the cerebellum is also involved in attention and language as well as the fear and pleasure response.
The Blood Brain Barrier
The role of the blood-brain barrier is to strictly regulate what substances can get from the bloodstream into the tissue system of the brain. The blood-brain barrier helps block harmful substances, such as toxins and bacteria from entering the brain. At the same time, the brain also depends upon the delivery of hormones and nutrients like glucose and several amino acids, from elsewhere in the body. The blood-brain barrier is one of the reasons why it is difficult to develop chemotherapy drugs which can reach the brain.
Compounds that are fat-solublepass through cells that make up the blood-brain barrier easilly. They are transported through the cell wall of the vessel which is made up of a lipid bilayer. Larger molecules like glucose or insulin however have to be actively transported out of the blood vessel by proteins located in the blood vessel walls. This system allows the brain to be selective for desired molecules. Only those selected by these proteins are taken across the blood-brain barrier into the brain. Cells on the brain side of the barrier can control this transport by synthesising the proteins required. The more protein availiable the more molecules are transported and vice versa, this for example allows neurons to pull more glucose across the barrier when they are working hard.
Figure 11: Ordinary Capillary Blood Cell, with pores allowing large molecules to leave the capilary and reach cells. (Source: Jake Burton).
Figure 12: Brain Capillary Blood Cell with tight junctions preventing large molecules from leaving the cell without carrier mediated transport. (Source: Jake Burton).
CSF serves several purposes:
- Buoyancy: The actual mass of the human brain is about 1400 - 1500 grams and when suspended in the CSF it is equivalent to a mass of 25 - 50 grams. The structure and constitution of the brain makes it deform under its own weight. The CSF helps the brain to maintain its density without getting affected by its own weight. (Saladin, Kenneth et.al)
- Protection: CSF protects the brain tissue from physical injury, by providing a fluid buffer that acts as a shock absorber. (Wright, Ben et.al)
- Prevention of brain ischemia: Ischemia is a restriction in the blood supply to tissues causing shortage of oxygen and glucose resulting in tissues death. The prevention of brain ischemia is made by decreasing the amount of CSF in the limited space inside the skull. This decreases total intracranial pressure and facilitates blood perfusion.(Saladin, Kenneth et.al)
- Homeostasis: CSF flows throughout the inner ventricular system in the brain and is absorbed back into the bloodstream, rinsing the metabolic waste from the central nervous system through the blood–brain barrier. This allows for homeostatic regulation of the distribution of neuroendocrine factors, to which slight changes can cause problems or damage to the nervous system.(Saladin, Kenneth et.al)
- Clearing waste: CSF provides a mechanism for the removal of waste products from the brain (Wright, Ben et.al).
Tumour Types
There are over 100 different types of brain tumour. The main distinction made between brain tumours is whether they are primary or secondary. A primary brain tumour is one which has started in the brain or a related structure like the spinal cord. The starting location of a primary tumour is linked to age (Cancer Research UK, 2015b). In adults, most primary brain tumours start in the cerebrum, followed by the meninges (1 in 4) and the brains associated glands, such as the pituitary gland (1 in 10). For children, most tumours start in the cerebellum or the brain stem (6 in 10) with far fewer starting in the cerebrum (4 in 10).
Secondary brain tumours are cancers that have spread to the brain from somewhere else in the body. They may also be referred to as brain metastases because they are caused by another cancer metastasising to the brain. This is where cancerous cells break off from the primary cancer body and travel through the bloodstream to a new location where they begin to grow into new tumours. Most common cancers e.g., lung, breast, stomach, bowel and skin (melanoma) can spread to the brain.
As well as the primary/secondary division brain tumours can generally be classified into cancerous, or malignant and non-cancerous, or benign. This division is related to the concept of tumour grading. [link to later]. If it is not possible to take a biopsy (sample) of the tumour then may not be graded.
Malignant tumours are likely to have arisen from glial cells. These tumours are known as gliomas. If the tumour came from a specific type of cell rather than a mixture it may be given a more precise name. For example, astrocytomas develop from the type of glial cell known as astrocytes. Similarly, Oligodendrogliomas develop from a glial cell known as a Oligodendrocyte and Ependymomas from Ependymal cells.
Benign brain tumours may also arise from glial cells but also commonly develop in other parts of the brain. The table below gives a number of examples. Whilst these types of tumour may tend to be benign they can also be malignant.
Meningiomas | Tumours of the meninges, these are the membranes which cover the brain. |
Acoustic neuromas (also known as Vestibular schwannomas) | Tumours of the acoustic nerve which develop from the Schwann cells on that nerve. |
Craniopharyngiomas | Tumours near the base of the brain. |
Haemangioblastomas | Tumours in the blood vessels of the brain. |
Pituitary adenomas | Tumours in the pituitary gland. |
Tumours of the pineal region and spinal cord or primitive neuroectodermal tumours may also appear as either malignant or benign.
It is also possible to get Lymphoma in the brain. Lymphoma are blood cell tumours which develop from a type of white blood cell called a lymphocyte. Lymphoma is treated differently to other brain tumours.
Astrocytoma
An astrocytoma is a glioma which originates in the astrocyte cells in the cerebrum (The Brain Tumour Charity, 2015). It is the most common glioma and can occur in most parts of the brain due to the abundance of their progenitor cells – astrocytes are the most abundant cells in the brain. An astrocytoma can be any grade on the 1-4 tumour grading scale. The grade of an astrocytoma is based on an assessment of the shape of the cells and their surroundings, e.g. the level of vascularisation (blood supply).
A grade 1 astrocytoma is sometimes called a pilocytic astrocytoma. This name is due to the shape of the cells which are ‘elongated and hair like’. These astrocytoma’s tend to occur in the cerebellum and the optic pathways. They are most common in children and young adults.
A grade 2 astrocytoma is sometimes called a diffuse astrocytoma. The name is a reference to the fact that the tumour does not have well defined edges. Like grade 1 astrocytoma’s they are slow growing but may return more aggressively after treatment. They are most common in adults with men more likely to get this type of cancer than women.
A grade 3 astrocytoma is sometimes called an anaplastic astrocytoma. They are so named because the cells divide rapidly and look very dissimilar to normal astrocytes. This type of astrocytoma is most common in older adults, aged 30 to 70 and again affects males more often than females. A grade 3 astrocytoma often recurs after the treatment of the more advanced grade 4 astrocytoma or ‘gliobastoma’.
Grade 4 astrocytomas are called glioblastomas or glioblastoma multiforme, often shortened to GBM. There are two types of glioblastoma, primary and secondary. Similar to the primary/secondary brain tumour division this classification refers to whether the first occurrence of the tumour is as a glioblastoma (primary) or whether it is a development from a lower grade astrocytoma (secondary).
Astrocytoma Genetics
Research (The Brain Tumour Charity, 2015) has found that grade 1 astrocytomas often have a mutation in the BRAF gene. This gene encodes a protein that is involved in carrying cell division signals (mitogenic signals) from the cell membrane to the nucleus (“BRAF_HUMAN Protein Information,” n.d.). Defects in the BRAF gene are found in a number of cancers including astrocytomas such as colorectal cancer, lung cancer and non-hodgkin lymphoma. In addition to mutations in the BRAF gene, grade 1 astrocytomas also have mutations in a gene called NF1 which encodes a protein called Neurofibromin (“NF1_HUMAN Protein Information,” n.d.). Mutations in this gene also occur in Leukemia and colorectal cancer.
Grade 2 astrocytomas often have mutations in the TP53 gene. This gene codes for a protein, ‘Cellular tumour antigen p53’, a protein specifically involved in tumour suppression by preventing growth or triggering cell death (apoptosis) (“P53_HUMAN Protein Information,” n.d.). Because of its direct role in controlling tumour growth TP53 is found frequently mutated or inactivated in about 60% of cancers.
Another common mutation occurs in the gene for ‘Platelet-derived growth factor receptor beta’, PDGFRB, which plays an essential role in the regulation of embryonic development, cell proliferation, survival, differentiation, chemotaxis and migration (“PDGFRB Protein Information,” n.d.). PDGFRB is a proto-oncogene, a protein whose normal cellular gene can be converted into a cancer-promoting oncogene (an oncogene is a gene that has the potential to cause cancer) by mutations.
Mutations in IDH1 are also common in gliomas of all grades.
Oligodendroglioma
Oligodendrogliomas are primary brain tumours. They develop from the type of glial cell known as an oligodendrocyte. These are the cells responsible for producing the insulating myelin covering of nerve cells in the brain. Most oligodendrogliomas occur in the frontal and temporal lobe. The symptoms exhibited by the patient depend on the location. Frontal lobe oligodendrogliomas cause gradual change in personality as well as weakness in muscles on one side of the body. Temporal lobe oligodendrogliomas can cause problems with speech and coordination and might affect memory.
Oligodendrocytes tend to be either grade 2, slow growing, or grade 3, malignant. This latter type are sometimes referred to as anaplastic oligodendrogliomas. Oligodendrogliomas may sometimes also contain astrocytes, the type of cells from which astrocytomas develop. Genetic testing can be used to differentiate the two types of tumour.
Ependymoma
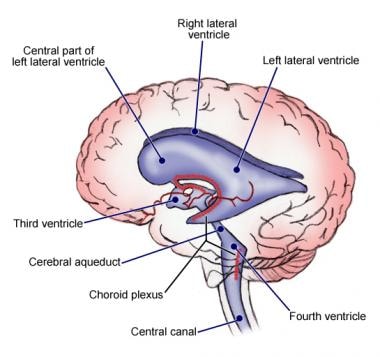
Ependymomas are a type of glioma originating from ependymal cells. These are the cells responsible for producing and circulating cerebral spinal fluid (CSF) and line the 4 fluid filled areas of the brain, the ventricles. This type of tumour is very rare, only 2 in 100 brain tumours are ependymomas. This type of tumour may be both low grade or high grade. As well as growing in the brain this type of tumour can also grow in the spinal cord. In some cases the tumour can spread into the CSF and through this into other areas of the lining of the brain and spinal cord.
CNS Lymphoma
Whilst lymphomas generally form in the lymph nodes around the body it is possible for them to form in the central nervous system. Lymphoma is cancer of the lymph cells which are a type of white blood cell and form part of the bodies immune system. This type of tumour is far more common in those with a weak immune system.
CNS Lymphoma can either be a mass that presses on the brain or spinal cord from outside or one that spreads within the meninges. CNS Lympohmas are usually high-grade and a type of lymphoma called diffuse large B cell non-Hodgkin lymphoma. In contrast to other types of brain tumour, CNS lymphoma is treated mainly with chemotherapy rather than radiotherapy. However, some patients have radiotherapy following their chemotherapy.
Meningioma
Meningiomas are brain tumours which start in the protective lining of the brain, the meninges. They are usually low-grade and slow growing. Meningiomas can only be graded at 1, 2 or 3. As such, the ‘watch-and-wait’ approach, using steroids to control the symptoms may be taken and more invasive treatments delayed to a later date. The cause of meningiomas is unknown but previous radiation to the head or a genetic condition called type II neurofibromatosis may be risk factors. Some meningiomas have hormone receptors that interact with certain hormones like progesterone, androgen and oestrogen and it has been observed that meningiomas can grow faster during pregnancy.
Acoustic Neuroma
These are tumours which start in the acoustic nerve. The acoustic nerve is responsible for controlling both our hearing and balance. The acoustic nerve is myelinated by Schwann cells, rather than oligodendrocytes as neurons in the brain so this tumour is a type of schwannoma. Acoustic neuromas are benign and slow growing. Whilst they can cause problems by pressing on surrounding tissue they cannot spread into the brain itself. Acoustic neuromas are most common in people in their 40s to 60s. As with Meningioma previous radiation treatment to the head and type II neurofibromatosis are risk factors.
Haeomangioblastoma
Haeomangioblastomas are tumours which develop from the cells that line the blood vessels in the brain and spinal cord. In particular, they tend to develop in the cerebellum and less frequently in the brain stem and spinal cord. This is a slow growing, benign form of tumour. Patients with a genetic condition known as von-Hippel-Lindau disease have a greater risk of developing this tumour.
Medulloblastoma
Medulloblastomas are cancerous tumours which develop in the cerebellum and other areas in the posterior fossa. The posterior fossa is a small space at the back of the skull containing the cerebellum, brain stem and 4th ventricle. These are the most common high-grade childhood tumours and are commonly found in children between 3 and 8 years of age. This type of tumour occurs less frequently in adults, and tends to affect people under the age of 45.
The 4th ventricle contains CSF and it is possible for a medulloblastoma to spread through this to other parts of the brain or spinal cord. Commonly these tumours block the flow of CSF which leads to a build up of CSF in the ventricles in the brain and increased pressure on the brain itself. A condition which is called hydrocephalus.
Pituitary Gland Tumours
The pituitary gland is located off of the bottom of the hypothalamus at the base of the brain. Tumours which start here are called adenomas and are usually benign. Tumours of the pituitary gland are of two types, those that produce hormones (secreting) and those which do not (non-secreting). The symptoms of pituitary gland tumours are slightly different from other types of brain tumour as they tend to be caused by the over or under-production of certain hormones. Pituitary gland tumours can also press on the optic nerve, which is located in close proximity to the pituitary gland causing headaches and vision problems.
Symptoms caused by a change in hormone levels often take a long time to develop. Prolactin secreting tumours are the most common type of secreting tumour. Prolactin is the hormone which stimulates breast milk production. Women with this type of tumour may produce small amounts of breast milk and their periods may stop. In men over-secretion of prolactin results in impotence and reduced libido. Prolactin secreting tumours can also cause infertility in both women and men.
Any of the hormones produced by the pituitary gland can be over produced by a tumour with differing symptoms. Growth hormone secreting tumours lead to abnormal growth and enlargement of the hands, feet, lower jaw and brows among other symptoms. TSH-secreting tumours cause weight loss, irregular heart beat and anxiety among other symptoms. ACTH-secreting tumours cause the symptoms of Cushing’s syndrome which includes weight gain, depression, high blood pressure among others.
Pineal Gland Tumours & Germ Cell Tumours
These tumours start in the pineal gland in the centre of the brain and are rare. There are different types of pineal region tumours. General pineal gland tumours develop from the cells of the pineal gland. These are pineocytomas and pineoblastomas. Blastomas are cancers derived precursor cells or embryonic tissue and are more common in children than adults whilst cytomas are general cell tumours.
The more common type of pineal region tumour is a germ cell tumour. As the name suggests these are tumour which develop from germ cells. Germ cells are the cells which give rise to gametes, in humans the gametes are the egg and sperm cells. During the fetal period (the final stage in a unborn baby’s development) these cells can become trapped in the brain usually near the pineal and pituitary gland where they develop into tumours. There are two main groups of germ cell tumour: germinomas and nongerminomatous tumours. These tend to grow faster than non-germ cell tumours but respond well to treatment. Pineoblastomas are sometimes classified as PNETs.
PNET Primitive Neuro-Ectodermal Tumour
PNETs develop from the neuroectoderm which is the embryonic tissue which develops into the nervous system. The cells which go on to form PNETs have not developed in differentiated in the normal way into a neuron. PNETs are rare and usually occur in children and those under 25 although the incidence decreases with age. PNETs tend to be high grade and fast growing.
PNETs can occur in both the brain and the spine and can be divided into two groups depending on their location in the brain. Either above or below the tentorium, the membrane separating the cerebellum and the brain lobes. Supratentorial means above the tentorium and infratentorial means below the tentorium. The latter type are more common and include medulloblastomas and pineoblastomas which are types of PNET.
Choroid plexus carcinoma
A carcinoma is a type of cancer developing from epithelial cells i.e., the cells lining the bodies cavities, blood vessels and organs. In the case of Choroid plexus carcinoma these cells are the ones lining the Choroid plexus. These are the regions in the brains ventricles which produce the CSF, the cells here are modified ependymal cells. These tumours can block the cerebro-spinal fluid from circulating and draining leading to increased pressure on the brain. Choroid plexus carcinomas are grade 3 tumours and are fast growing. They most often occur in very young children, approx. 1 years old. There is an association with a genetic condition called Li-Fraumeni syndrome.
A related tumour occurring in the same location but which has a lower grade (1 or 2) are choroid plexus papillomas. Papillomas are benign tumours which grow in an outwardly projecting finger-like fronds.
Craniopharyngioma
A craniopharyngioma is a benign brain tumour found at the base of the brain, near to the pituitary gland. Due to their location they are often associated with symptoms such as problematic vision and growth. They may also caused hydrocephalus, diabetes and personality changes. This type of tumour is most often diagnosed in children 5 to 15 but can present much later in adults around 45-60.
DIPG – Diffuse Intrinsic Pontine Glioma
DIPGs are high grade brain tumours affecting children which originate in the pons, a part of the brainstem which connects the medulla oblongta and midbrain.
Brain Tumour Symptoms
Because of the brain’s involvement in a wide range of functions symptoms are equally varied. It is possible not to have any symptoms to begin with or have them only develop slowly over time. Whilst the exact symptoms depend on the part of the brain that is affected there are a number of common symptoms. These include (NHS, 2017):
- Severe and long lasting headaches
Brain tumour associated headaches are usually throbbing headaches and are worse in the morning, or after you have been lying down for a prolonged period. When you stand the build-up of CSF begins to drain and the pain or intensity of the headache may reduce.
- Seizures
Small seizures are more common than severe seizures in people with brain tumours. During a small seizure parts of the body by twitch and the person can experience changes in sensation. Whilst you do not lose consciousness during such an event the patient may experience moments of ‘absence’. Contrastingly, during a serious seizure the person will lose consciousness for the entire duration of the fit and the whole-body twitches.
- Long lasting nausea, vomiting and drowsiness.
Nausea associated with brain tumours may be worse in the morning or if you suddenly change posture. The drowsiness symptom usually occurs later, when the tumour has grown a fair amount. As the intracranial pressure increases people tend to sleep more than normal and may even fall asleep during the day.
- Mental or behavioural changes, for example changes in personality (e.g. becoming short tempered) or memory problems (e.g. increased forgetfulness)
- Progressive weakness or paralysis on one side of the body
- Problems with vision or speech
Vision problems may involve blurring of vision or complete temporary loss of vision. This is known as ‘greying out’. These visual effects will often occur when you suddenly stand up.
Brain tumours are comparatively rare and the above symptoms can be caused by several other conditions as well. Brain tumours can affect anyone but are most common in older adults. Most of these symptoms are caused by raised intracranial i.e., in the skull, pressure. The build-up of this pressure may occur quickly or over a long period of time.
As brain tumours grow they may begin to affect normal brain function. The tumours location in the brain determines which functions are disturbed and the symptoms that occur as a result. For instance, brain tumours in the frontal lobe cause difficulty with concentrating, speaking, controlling emotions and learning new information. Whilst those in the temporal lobe may produce difficulty hearing, speaking, learning, or empathising.
Tumours in other areas may cause more visible symptoms due to their location. Tumours which are found in the parietal lobe can lead to difficulty coordinating movements, reduced spatial awareness and, problems understanding speech, writing or reading. A patients’ ability to identify objects or colours may be affected by tumours of the occipital lobe. Vision problems can also be caused by tumours in the brain stem which may also cause facial weakness, walking difficulties and difficulty with speaking or swallowing.
Finally, tumours in the cerebellum may produce symptoms including: loss of balance, vomiting, stuff neck, loss of dexterity/co-ordination, difficulty walking and flickering of the eyes.
Brain tumour symptoms differ slightly in children, though a number are common to adults as well. They include: persistent vomiting or nausea, recurring headache, abnormal eye movements, fits or seizures, behaviour change, reduced balance and co-ordination, blurred vision, abnormal head position (a desire to have the head titled for instance) and, delayed or arrested puberty. Additional symptoms include reduced consciousness, abnormal growth (i.e., growth behind what is expected for their peer group, or age) and diabetes insipidus.
Diabetes insipidus differs from what is often referred to as just ‘diabetes’ but is technically Diabetes mellitus, the disease of having high blood sugar levels for a prolonged period. Instead Diabetes insipidus is a condition characterised by excessive thirst and excretion of large amounts of severely diluted urine. It can be caused by kidney dysfunction but in the case of brain tumours is caused by a disturbance in the production of antidiuretic hormone (ADH) in the pituitary gland. ADH regulates the body’s retention of water.
Brain Tumour Diagnosis
There are several steps involved in a brain tumour diagnosis. Patients presenting with symptoms indicative of a brain tumour in primary care will often be referred to a specialist e.g., a neurologist for diagnosis. In addition to a general examination of the patients’ health (history, physical examination) a neurological examination is also conducted. This involves testing vision, hearing, muscle strength, coordination and reflexes. Swelling of the optical disc, a circular area at the back of the eye where it connects to the optic nerve may be indicative of a brain tumour because it shows raised pressure inside the skull.
Following this examination, several imaging studies may be performed to establish whether a tumour is present. Both MRI (magnetic resonance imaging) and CT (computer tomography) imaging techniques are used in clinical practice. If a patient is treated in an emergency setting e.g., after a seizure the imaging may be performed before the more general neurological examination.
If the MRI or CT scan identifies a tumour a full body scan is carried out to establish if the tumour is a primary or secondary brain tumour. Provided that the tumour is operable a biopsy of the tumour tissue will be taken to allow the type to be identified (a biopsy is a surgical procedure to remove a small sample of the tumor for examination under a microscope). This may be performed at the same time as a surgical removal of either part of, or the entire tumour. This is called a resection. The biopsy sample is analysed by a neuropathologist who will identify the type of tumour and assign it a grade.
Occasionally biomarker testing might be performed to help identify the tumour. However, this practice is not widespread. These tests can also assist in tumour grading and treatment planning. For example, the MGMT methylation test can be used to predict the effectiveness of chemotherapy. A number of biomarker tests are summarised below.
Other Tests
A lumbar puncture is a procedure in which a doctor uses a needle to take a sample of cerebrospinal fluid (CSF) to look for tumor cells, blood, or tumor markers. Occasionally blood or urine tests may also be performed to look for signs of brain tumours, for example when they lead to the overproduction of some hormones.
Tumour Grading
There are 4 grades which describe the speed at which a tumour is likely to grow at (Cancer Research UK, 2015). Grades 1 and 2 are occasionally referred to as low grade or benign whilst grades 3 and 4 are referred to as high grade or malignant. It is possible for a benign tumour to become malignant (2 to 3) and for a malignant tumour to become even more so (3 to 4). Because some regions of the brain are very important even benign brain tumours can be life threatening.
Benign tumours are generally slow growing and less likely to spread. They are also less likely to return after surgery. In contrast, malignant tumours are relatively fast growing and likely to spread. They may also come back after surgery which may mean that radiotherapy or chemotherapy is needed to control the tumour.
Bibliography
Boots-Sprenger, S.H.E., Sijben, A., Rijntjes, J., Tops, B.B.J., Idema, A.J., Rivera, A.L., Bleeker, F.E., Gijtenbeek, A.M., Diefes, K., Heathcock, L., Aldape, K.D., Jeuken, J.W.M., Wesseling, P., 2013. Significance of complete 1p/19q co-deletion, IDH1 mutation and MGMT promoter methylation in gliomas: use with caution. Mod Pathol 26, 922–929. doi:10.1038/modpathol.2012.166
Cancer Research UK, 2015. Brain (and spinal cord) tumours: Grades [WWW Document]. URL http://www.cancerresearchuk.org/about-cancer/brain-tumours/grades (accessed 5.12.17).
Cancer Research UK, 2015b. Brain (and spinal cord) tumours: Primary and secondary brain tumours [WWW Document]. URL http://www.cancerresearchuk.org/about-cancer/brain-tumours/types/primary-secondary-tumours (accessed 5.12.17).
Clark, K.H., Villano, J.L., Nikiforova, M.N., Hamilton, R.L., Horbinski, C., 2013. 1p/19q testing has no significance in the workup of glioblastomas. Neuropathol Appl Neurobiol 39, 706–717. doi:10.1111/nan.12031
The Brain Tumour Charity, 2014. Biomarkers [WWW Document]. URL https://www.thebraintumourcharity.org/media/filer_public/a7/47/a7476307-1370-4d89-8c41-bbb4025532df/biomarkers_v20a.pdf (accessed 5.12.17).
Michael Demitri, Types of brain imaging techniques. [Online] Available at : https://psychcentral.com/lib/types-of-brain-imaging-techniques/
Hermoye, Laurent. Diffusion Tensor Imaging [Online] Available at: http://www.imagilys.com/diffusion-tensor-imaging-dti/



















Kommentar
Izabela Horvath sagt:
17. Mai 2017WOW! Nice and extensive info! And I really like that you related symptoms to tumour type ! Overall very neatly organised - maybe the bibliography could be a bit more..cleaned up. Oh, and watch out for 'Unknown attachment'(Fig 8 & 9) Otherwise, impressive job!