Navigation in surgery is a wide concept, which depending on the clinical challenge and application, may have different interpretations. It comprises the group of techniques that respond to important anatomical orientation questions, like "Where is the anatomical target", "How can it be reached safely", "Where I am anatomically located" and "Where and how should the implant be positioned". Moreover, surgical navigation is also used as a measurement tool and information centre that provides the surgeons the right information at the right time. Surgical navigation is frequently called Stereotactic surgery or stereotaxy.
The basic principle of navigated surgery is to see the tip of a pointer in an image space. Then the relationship between the device space and the image space is established (registration or calibration of the navigation device). This relationship is a transformation matrix that maps the coordinates of any point between the image and the device spaces. Finally, a linkage between digital image data and anatomical structure is obtained. Today navigation systems provide approximately 2mm accuracy.
Neuronavigation
In neurosurgery, the localisation and delineation of extent of lesions are critical for safely resect brain and spinal cord tumours. The location of small intracranial tumours is the most frequent application of navigated technology for adults and children. There are different options for navigation in brain surgery. These are three examples where navigation is used.
Endoscopic Skull Based Surgery (ESBS)
Skull based surgeries are procedures to remove both benign and cancerous growths, as well as abnormalities on the underside of the brain, the skull base or the top few vertebrae of the spinal column. Due to the fact that this area is extremely difficult to access, it is usually done via a minimally invasive endoscopic procedure.
The instruments are inserted through the natural openings of the skull, i.e. nose and mouth, of by a small hole above the eyebrow.
Brain Biopsy
In these kind of surgical procedures, small fiducial markers are stuck to different parts of the scalp, providing reference landmarks. Once the patient is asleep, and the fiducial points are registered by cameras into the computerised navigation system, a biopsy needle with is introduced to the target and the biopsy samples are obtained. X-Rays or CT are used to locate the lesion and ensure the needle is placed directly into the abnormal area.
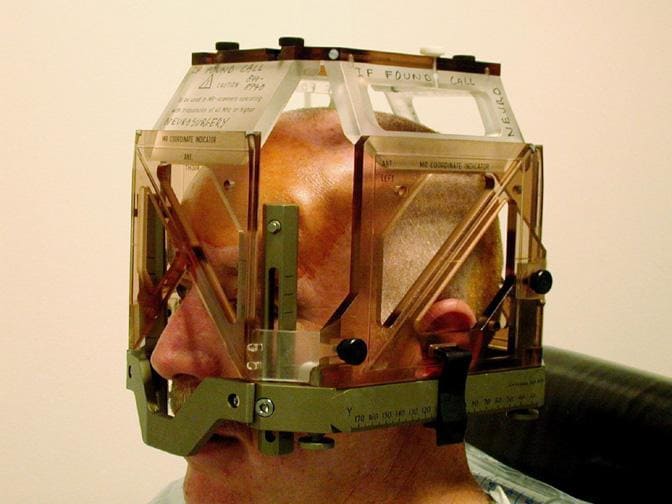
Frame based and Frameless Navigation
In the last two examples, two different of ways to provide a reference system in order to map the target points have been introduced. Stereotactic head frame and fiducial markers, i.e. frame based and frameless navigation.
Frame Based Navigation
A light-weight frame is attached to the head using local anesthesia. Then the head is imaged by either CT, MR or angiography to identify the target in relationship to the external frame. Since both frame and target are visible in the images, the distance of the target from the reference points on the frame can be measure in three dimensions. Therefore, the surgical utensils attached to the head frame can be adjusted to the 3D coordinates of the target, which can be now accurately approached by the surgeon.
This technique has been long considered "the gold standard for sampling intracranial lesions". Due to the rigid frame it provides excellent target precision. However the usage is limited by the following aspects:
- The frame's bulkiness.
- Patient's discomfort.
- The calculations involved with defining the stereotactic entry points
- Possible prolonged surgical time
- Risk of postoperative infection at the frame's fixture points.
- Frames can obstruct the operative field.
- They limit the surgeon to targets along a straight-line trajectory.
Therefore, there is ongoing research on developing easy to use frameless techniques with a comparable diagnostic yield.
Frameless Navigation
Frameless navigation was first introduced in the 1980s. Since then, several different frameless stereotactic devices have been developed. However, as well as in frame-based devices, all systems must define points in both the image and the patient, as be able to map the two groups to each other.
Among the frameless navigation devices there are two methods for defining points: surface-based and point-based.
- Surface-based registration: It fits a set of points from the contours of one image to a surface model from contours of the patient's head, or from other images. This system allows retrospective registration from prior image. Nevertheless, it is less accurate.
- Point-based registration: This method involves selecting corresponding points in different images and on the patient. The coordinates of each set of points are defined, then a geometric transformation is calculated between them. These points may be anatomical landmarks or artificial markers. Anatomical landmarks allow retrospective registration with existing images. However, artificial extrinsic markers do not allow this but they are commonly used in stereotactic guidance. Extrinsic point registration allows greater accuracy than other registration methods. In addition, a calculation of the accuracy for each registration attempt in possible, giving the user a quantitated degree of accuracy. As long as a detectable marker can be manufactured, registration can theoretically be performed with any imaging modality. There are two types of extrinsic markers, also called fiducial markers:
- Mobile markers: they are taped, glued or affixed to the patient's skin. Their advantages rely on ease and speed of application. They are however prone to error because the skin may move relative to the skull and intracranial contents during registration.
- Rigid markers: They are anchored to the patient's skull. They are called fiducial markers. The problem with movement previously mentioned is potentially eliminated, but they are more difficult to apply and more uncomfortable to the patient.
Indeed, one of the drawbacks of frameless navigation with external fiducial markers, is to accurately position the markers. There has been research and development of algorithms to find an optimal fiducial configuration in order to reduce the misalignment.
From left to right. MRI scan showing circular fiducial markers on the forehead and near the eye [5] . 3D model of the optimal fiducial markers locations. [6]
These markers, once being placed have to been "seen" by the tracking device. There are two techniques to track them: optical and electromagnetic tracking.
- Optical tracking: It uses infrared sensors in combination with light-emitting structures or light reflectors that are fixed to the patient's head, via a headband strap or sticker, and then fixed to a handheld probe. Both headband and instrument must be detected by the system's camera in order to track where the surgeon's instruments are.
- Electromagnetic tracking: It uses reference points on a device attached to the patient's head with a plastic mask with metallic beads or a headband, and a wired instrument for the surgeon. These systems do not have to be "seen" by the computer, therefore it does not matter whether there are other devices and/or equipment between the computer and the patient. However, metals placed within the electromagnetic field may cause inaccuracies.
A further description and comparison of these methods will be explained in below.
As is has already been mentioned, both frame based and frameless stereotactic techniques use preoperative images with a registered probe to access the target tissue. Therefore they both suffer from the same drawback: there is no real-time feedback confirming that the needle is in fact in the target tissue. Intraoperative brain shifting, CSF loss, or technical issues may lead to a potential misalignment between the image guide and the actual brain configuration during the operation.
Furthermore, there are recent studies that plan to integrate the advantages of both frame-based and frameless stereotactic navigation into a single device. In [8] they propose a head-mounted robot-guided approach that combines the stability of a bone-mounted setup with the flexibility and tolerability of a frameless system. The human interference, such as manual parameter settings and calibration, is reduced and thus multiple trajectories to reach the target. Moreover, the patients don't experience permanent deficits nor infections after surgery. If you want to know more about this study, please click here.
Stereotactic Radiosurgery
Stereotactic radiosurgery (SRS) uses many focused radiation beams to treat tumours among other abnormalities, specially in the brain and the neck. Instead of incision, it uese 3D imaging to target high doses of radiation to the affected area so the impact is minimum on the surrounding healthy tissue. The DNA of the targeted cells is damaged by the radiation beam, and they lose the ability of reproduction and the tumour shrinks. It usually needs only one session.
There are three main types of technology to deliver the radiation:
- Linear Accelerator (LINAC): it uses high energy X-Rays that are shaped to conform the shape of the patient's tumour. The beam is directed to the patient tumour shaped by a multileaf collimator in the head of the machine. Lasers are used to make sure the patient is in the right position. By moving the treatment couch and the gantry from which the beam comes, the radiation can be delivered to the tumour at any angle.
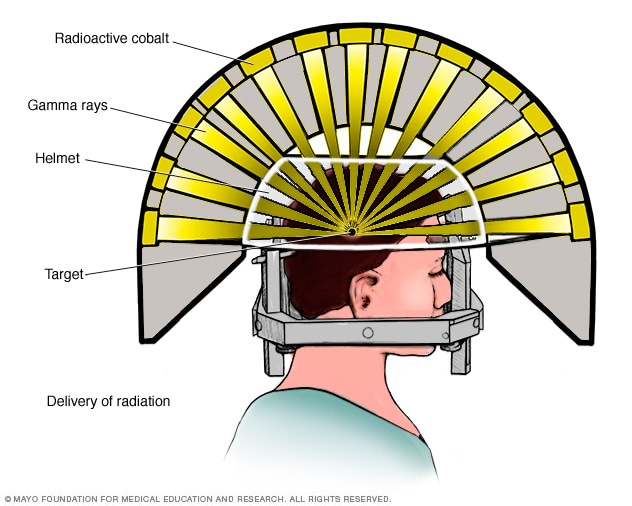
Gamma Knife: it uses 192 or 201 small beams of gamma rays to target and treat the brain cancerous tissue and abnormalities. A stereotactic frame is attached to four points in the scalp and forehead of the patient with local anesthesia. One the frame is placed, the exact position of the lesion or tumour is determined using CT or MRI. Afterwards a helmet with several holes is placed over the head frame. The holes help to focus the radiation beams on the target.
However, there has been some improvements in developing a frameless Gamma Knife. The device has been developed by Elekta and it is called Leksell Gamma Knife Icon. It uses mask-based immobilisation, instead of the stereotactic frame. It is capable of treating virtually any target in the brain, regardless of type, location or volume. Sometimes the target area is not very small and it is not near a significant organ, therefore the extreme accuracy of the head frame is not needed, and the mask provides the enough accuracy by making the patient more comfortable and avoiding future side effects from the head frame.- Proton beam: It is the newest technology, and it is only available in a few centres in the US. It uses a cyclotron or synchrotron to energise protons. Then they are extracted and directed with magnetic fields towards the tumour. The penetration depth is related to their energy and it can be controlled to precisely match the location of the tumour. Protons deliver the majority of their energy at a very narrow area within the body. This property is the Bragg Peak, and it can be manipulated to deliver the desired radiation dose to the tumour without any exit dose beyond the tumour. In comparison with the other techniques, the radiation dose to the healthy tissue is thus reduced.
Elekta Unity MR-linac devices. It combines MR scan with LINAC. It produces diagnostic level image quality during the therapy sessions. [10]
From left to right. Gamma Knife delivery of radiation [9] Elekta Gamma Knife device [11]. Leksell Gamma Knife Icon device. [14]
Typical treatment plan for proton therapy. The dashed blue line is the therapeutic radiation distribution. The SOBP is the sum of several individual Bragg peaks (thin blue lines) at staggered depths. As comparison, the depth-dose plot of an x-ray beam is provided (red line). The pink area is the additional doses of x-ray radiotherapy. [16]
Electromagnetic vs Optical Tracking Systems
As is has been introduced before, there are two main types of navigation systems available in the market today: Optical or infrared and electromagnetic systems. Both technologies have the same functions, but the technology that provides de information is different. In all cases, there will be either a head mask or head frame attached to the patient.
| Electromagnetic | Optical | ||
| Advantages | Drawbacks | Advantages | Drawbacks |
| Automatic CT/MRI Fusion | Interference with other metal instruments | Automatic CT/MRI fusion | Headset with reference frame is bulky and can interfere with accessibility |
| Intraoperative distance control | Interference with cardiac pacemakers and cochlear implants | Intraoperative distance control | Needs line of sight with the cameras and the infrared diodes and sensors |
| Electromagnetic field generator can be integrated into operative table and be detachable | Cables attached to electromagnetic tracking sensors | Real time display of instrument | Difficulty to change from endoscope to microscope if the equipment interferes with line of sight. |
| Headset sensor detachable and easily sterilised | Electromagnetic image guided navigational tracking system. [17] | Integration of endoscopic video image | Optical image guided navigation tracking system. [17] |
| All-in-one equipment in operation theater | Wireless probe available | ||
| No need of line of sight | No alteration in operation theatre setup | ||
| Tracking not affected by titanium | Frameless, no rigid fixation to operation table | ||
| Automatic instrument check upon registration | Active and passive tracking system, i.e. free hand probe sensed by passive system | ||
Comparative table of both optical and electromagnetic systems.
References
http://www.hopkinsmedicine.org/healthlibrary/test_procedures/neurological/skull_base_surgery_135,43/
[3] http://emedicine.medscape.com/article/1153743-overview
http://www.aans.org/Patients/Neurosurgical-Conditions-and-Treatments/Stereotactic-Brain-Biopsy
[6] http://medicaldevices.asmedigitalcollection.asme.org/article.aspx?articleid=1451820 http://medicaldevices.asmedigitalcollection.asme.org/article.aspx?articleid=1451820
[8] F. Grimm, G. Naros. Blurring the boundaries between frame-based and frameless stereotaxy: feasibility study for brain biopsies performed with the used of a head-mounted robot. Journal of Neurosurgery. Sep 2015 / Vol. 123 / No. 3 / Pages 737-742. Available here.
[10] https://www.medgadget.com/2017/05/elekta-introduces-unity-the-first-high-field-mr-linac-system.html
[11] https://www.elekta.com/patients/gammaknife-treatment-process/#sectionId6
[12] http://www.massgeneral.org/radiationoncology/BurrProtonCenter.aspx
[13] https://www.ucsfhealth.org/treatments/gamma_knife/
[14] https://www.careforthebrain.com/
[15] https://wavelength.elekta.com/2016/03/a-new-frameless-frontier/
[16] https://www.revolvy.com/topic/Proton%20therapy&item_type=topic
[17] Z. M. Samarakkody, B. Abdullah. The use of image guided navigational tracking systems for endoscopic sinus surgery and skull base surgery: A review. Egyptian Journal of Ear, Nose, Throat and Allied Sciences. Nov 2016 / Vol 17 / Issue 3 / Pages 133-137. Available here.