In this page an overview of the state of art according to animal trials will be presented. Firstly an introduction about the current regulation and the differences in countries around the globe. Finally, arguments against and in favour of performing research tests with animals will be introduced. The arguments will be based on real data and no opinion will be presented.
Regulation of Animal Trials
As all regulations, there are some variations about the legislation depending on the country of expedition. Here are three examples of current regulation.
Animal Welfare Act (7 U.S.C. § 2131) [1] [2] [3]
The Animal Welfare Act (AWA) is the only US federal law that covers animals in research, exhibition, transport and by dealers. Other laws, policies and guidelines may include additional species coverage or specifications for animal care and use, but the AWA is the minimum acceptable standard.
The AWA was first established in 1966 in response to a growing concern for dogs and cats used in research, particularly with regards to a large number of reported thefts of cats and dogs for use in research institutions. Since then, some amendments have been incorporated to the law, such as Improved Standards for Laboratory Animals Act (1985), Protection of Pets (1990) and Animal Fighting Prohibition Enforcement Act (2007).
The AWA it is enforced by the following organisms:
- U.S. Department of Agriculture (USDA): enacts regulations to implement the provisions of the AWA.
- Animal and Plant Health Inspection Service (APHIS): as part of the USDA, it conducts annual inspections of all licensed facilities and generally oversees compliance.
The AWA creates a regulatory network administrated by the USDA covering many commercial uses of many animals. Here is summary of the main topics addressed:
- Permits are required to buy and sell listed animals, or register for their use by dealers, exhibitors and research facilities that use listed animals. Pet owners, agricultural use and retail pet stores are exempted from this law.
- Regulations on how the animals may enter the controlled chain of commerce.
- Regulations on the environmental conditions under which the animals are kept.
- Research facilities must purchase listed animals only from licensed dealers.
- Research facilities must create an Animal Care Committee to review the animal use in their facility and inspect the animal housing facilities.
- Research facilities much abide by legal restriction on the imposition of pain during research.
- Research facilities must comply with extensive regulations concerning the housing and care of animals used in research.
- It is illegal for any person to knowingly sponsor or exhibit animals in any animal fighting venture to which any animal was moved in interstate or foreign commerce.
Here it can be found the Animal Welfare Act and Regulations "Blue Book" published by the USDA in September 2013.
However, one of the main concerns and controversy topics is the coverage of the AWA. It doesn't cover every type of animal used in every type of activity. This is its coverage:
- Farm animals used for food or fiber
- Coldblooded species (amphibians and reptiles)
- Horses not used for research purposes.
- Invertebrates
- Rats of the genus Rattus and mice of the genus Mus that are bred in use for research.
- Birds are covered under the AWA but the regulatory standards have not yet been established.
Animal Welfare Europe (Directive 2010/63/EU) [4] [5]
The last update on the protection of animals used for scientific purposes was adopted on 22 September 2010 (Directive 2010/63/EU revising Directive 86/609/EEC). The Directive is based on the Three R's principle: Replace, Reduce and Refine the use of animals for scientific purposes. The main changes are a wider scope and the inclusion of foetuses of mammalian species in the last trimester of development and cephalopods, as well as animal used for basic research, higher education and training. The use of great apes (chimpanzees, bonobos, gorillas and orangutans) is forbidden.
These Three R's principle previously mentioned are a set of principles that researchers are encouraged to follow in order to reduce the impact of animals in research. They can be summarise as follows:
- Reduction: Reducing the number of animals used in experiments by:
- Improving the techniques to perform the experiments
- Improving the techniques to analyse the data
- Sharing information with fellow researchers.
- Refinement: Refining the experiment and/or the way animals are taken care of in order to reduce their suffering by:
- Using less invasive techniques
- Better medical care
- Better living conditions
- Replacement: replacing experiments on animals with alternatives techniques such as:
- Using cell cultures instead of whole animals
- Human volunteers
- Epidemiological studies
- Computer models.
Furthermore, it lays down minimum standards for housing and care, regulated the animal use through a systematic project evaluation that requires inter alia assessment of pain, suffering distress and lasting harm caused to the animals. It requires regular risk-based inspections and improves the transparency through a publication of non-technical project summaries and/or retrospective assessment. The development, validation and implementation of alternative methods is promoted now by the establishment of a Union reference laboratory for the validation of alternative methods supported by laboratories within the Member States, while requiring the Member States to promote alternative methods at a national level. The use of the animals in research is authorised only in cases where no satisfactory substitute method exists. The projects may not begin until it is been demonstrated that the use of the animals is justified and that the expected advantages outweigh the harm caused to the animals. Animals may be killed only by a person with the required skills in the establishment of the breeder, supplier or user.
Animal testing can only be authorised in procedures which purpose is:
- Basic research.
- Translational and Applied research aimed at the prevention, prophylaxis, diagnosis or treatment of human or animal diseases.
- Development, manufacture or testing of the quality, effectiveness and safety of drugs, foodstuffs and feed-stuffs.
- Protection of the natural environment in the interest of both human and animal health.
- Higher education or training.
- Forensic investigations.
Full legislation text here.
China [6]
In March 2016, is was known that Chinas was setting the first guidelines for animal laboratory treatment. It covers topics like euthanasia, pain management, transport and housing, as well as requirements for breeding facilities and personal training. One of the main drivers of this decision was the reluctancy of foreign scientists to engage in research involving animals with Chinese institutions if they are not covered by humane protections. Furthermore, there is a growing domestic opposition to the mistreatment of lab animals.
Controversy and Debate regarding Animal Trials
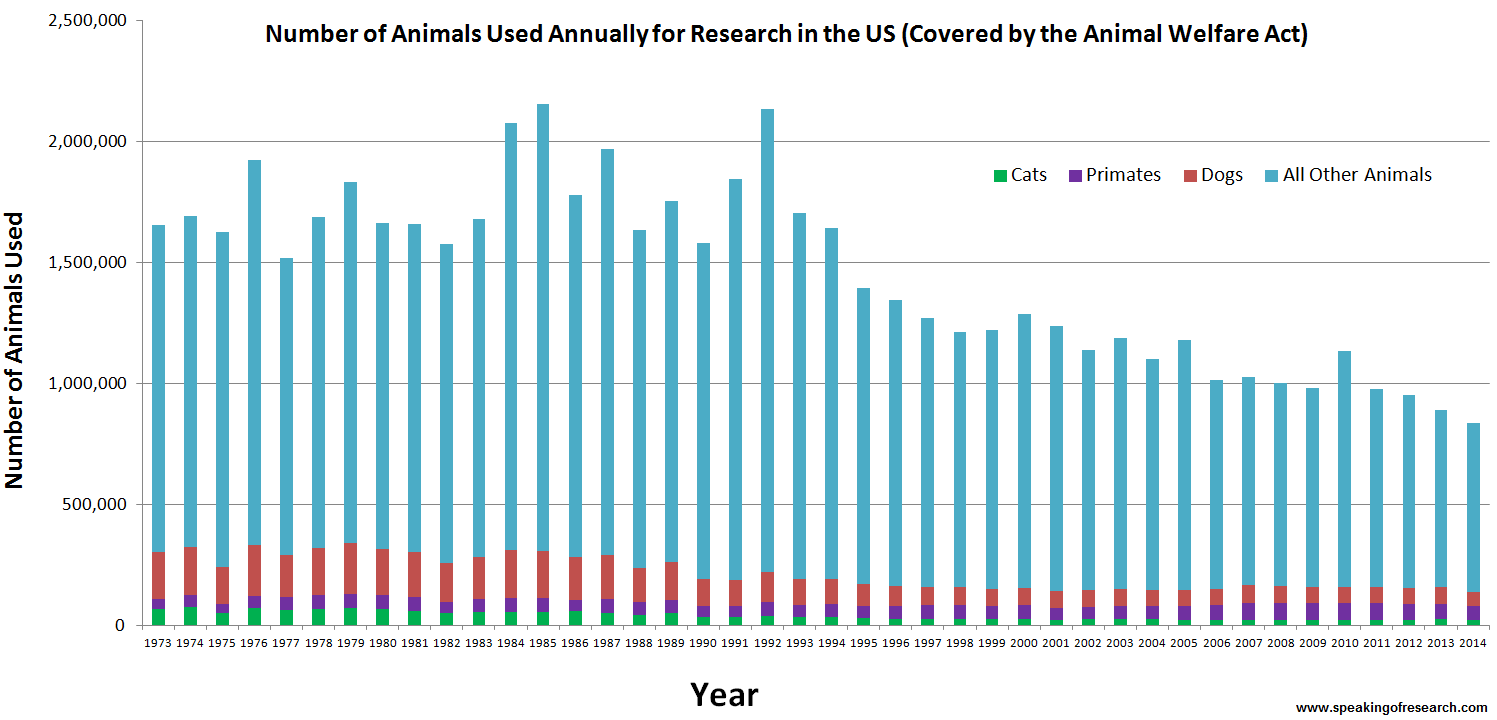
Only in the US, approximately 26 million animals are used every year for commercial and scientific testing. However, descriptions of the dissection of live animals have been found in ancient Greek writings from circa 500 BC. In May 2013, a poll found that 56% Americans say animal testing is morally accepted. This number decreased form 65% in 2011 and only 47% of people aged 18-34 say animal testing is morally acceptable. Furthermore, a study published in 2014 showed that the number of animals used in research is decreasing since the AWA establishment in 1966.
Statistics about the number of animals used in research from 1973-2014, separated by species. 'All other animals' include those species that are not covered by the AWA. [8]
2) There is no adequate alternative to testing on a living, whole-body system.
Human and animal systems are extremely complex. Cell cultures don't provide the opportunity to study interrelated processes in the endocrine, immune or central nervous system. Evaluating a drug requires a circulatory system to carry the medicine to different organs. Furthermore, conditions such as blindness and high blood pressure cannot be studied in tissue cultures. Computer models are only reliable when using accurate information from animal research for building the models, and they lack the power to simulate the complexity of the organs like the brain.
4) Animals must be used in cases when ethical considerations prevent the use of human subjects.
When testing toxicity of drugs, lives of human volunteers should not be at risk unnecessarily. It would be unethical to perform invasive experimental procedures and experiments involving genetic manipulation on human beings before the methods are tested on animals. According to the World Medical Association Declaration of Helsinki, human trials should be preceded by test on animals. To read more information about this declaration, please click here.
5) Animals themselves benefit from the results of animal testing.
Animal testing has been very important in saving endangered species from extinction, like the black-footed ferret, California condor and tamarins of Brazil. Koalas, ravaged by an epidemic of sexually transmitted chlamydia and now classified as endangered in some regions of Australia, are being tested with new chlamydia vaccines that may stall the animal's disappearance. Furthermore, the incidence number of diseases like rabies and tetanus has decreased.
Against Animal Trials
There are many organisations around globe fight animal testing. For example, People for the Ethical Treatment of Animals (PETA) is described as the most successful radical organisation in America. PETA seeks total animal liberation, meaning no meat or dairy, no aquariums, circuses, hunting, fishing, no fur or leather and of course no medical research. The number of donors and supporters has been increasing from the last years. In 2015 PETA almost collected $42 million in donations.
Another organisation is the European Coalition to End Animal Experiments (ECEAE). It was formed in 1990 to campaign for a ban on animal testing for cosmetics. After successfully achieving their objective, they lead the European campaign against all animal testings. Less radical than PETA, they have stakeholder status with official EU bodies which intersect with animal experiments. They campaign for humane, modern science and progressive legislation.
Furthermore, the "Lush Prize" rewards every year initiatives across science and campaigning that work to end or replace animal testing particularly in the area of toxicology research.
Logo of Lush Prize 2017 [17]
PETA Propaganda agains animal testing.
Ad of PETA with musician Dave Navarro "putting himself in the shoes of the animals who live a painful life and suffer an agonizing death for these archaic experiments". [16] These organisations often take advantage of a celebrity's face to influence the public opinion.
These are the main arguments against animal testing.
1) Animal testing is cruel and inhumane.
According to Humane Society International, animals that are used in experiments are often subjected to force feeding, forced inhalation, food and water deprivation, prolonged periods of physical restraint, infliction of burns and other wounds to study the healing process, infliction of pain to study its effects and remedies, and killing. For example, the Draize eye test is used by cosmetic companies to evaluate shampoos and other products irritation. It uses rabbits incapacitated in stocks with their eyelids held open by clips, sometimes for multiple days, so they cannot blink away the products being tested. The USDA makes an annual report of the animal usage. The report published in 2015 stated that 767,622 animals suffered pain during experiments. Please find here the report.
2) Alternative testing methods now exist that can replace the need for animals.
During the last years, there in an increasing research trend about alternative methods to animal testing. Some of these methods are:
- In vitro cell cultures: Almost every type of human and animal cell can be grown in the laboratory. Studying cell cultures in a petri dish can produce more relevant results as human cells can be used.
- Human tissue: both healthy and diseased tissues donated from human volunteers can provide a more relevant way to study human biology. Moreover there are companies like Episkin that provide artificial human skin made from sheets of human skin cells grown in test tubes or plastic walls, and can produce more useful results than testing chemicals on animal skin.
- Computer models: Nowadays, computer models of the heart, lungs, kidneys, skin, digestive and musculoskeletal systems already exit, and they can be used to conduct virtual experiments based on already existing information and mathematical data.
- Volunteer studies: The development of sophisticated scanning devices and recording techniques has made safer to study human volunteers. Furthermore there is a new technique called microdosing that measures how very small doses of potential new drugs behave in the human body. These microdoses are radiolabeled, injected into human volunteers and measured via blood samples with an accelerator mass spectrometer.
3) Animals are very different from human being and thus make poor test subjects.
For example, rats are the animals more used in vivisection, and as they have no gall bladder, they excrete bile very effectively. Many drugs are excreted via the bile, so it affects the half-life of the drug.
4) Drugs that pass animal tests are not necessarily safe
FDA reports that 92% of drugs approved for testing in humans after trials in animal, fail to receive approval for human use. This rate has increased since 1985, in spite of all the advances in modern medicine. For example, [19] in 2004 a drug called Vioxx for treating arthritis was withdrawn of the market after 3.800 product-liability and injury lawsuits were filled agains Merck, the pharmaceutical company that was in charge of selling this drug. According to the Physicians Committee for Responsible Medicine (PCRM), the company relied on animal studies, including those performed on African green monkeys to claim that Vioxx was safe, although previous clinical test showed that the drug increases the likelihood of heart diseases. To read the full article, please click here.
5) Animal test may mislead researchers into ignoring potential cures and treatments
As opposite in the previous arguments, there are chemicals that have been proven to be harmful to animals, but valuable when using in humans. For example aspirin is harmful for some animals like cats. Moreover, the stress experienced by the animals in the lab may reduce the reliability of the research results. Stress influences important variables like heart rate, blood pressure, muscular activity and hormone levels.
Bibliography
[1] https://www.nal.usda.gov/awic/animal-welfare-act
[3] https://www.aphis.usda.gov/aphis/ourfocus/animalwelfare/SA_AWA
[4] http://ec.europa.eu/environment/chemicals/lab_animals/legislation_en.htm
[5] http://eur-lex.europa.eu/legal-content/EN/LSU/?uri=CELEX:32010L0063
[6] http://www.sciencemag.org/news/2016/03/china-finally-setting-guidelines-treating-lab-animals
[7] http://animal-testing.procon.org/
[8] https://speakingofresearch.com/2015/07/09/usda-publishes-2014-animal-research-statistics/
[9] https://www.crueltyfreeinternational.org/why-we-do-it/alternatives-animal-testing
[10] http://www.thecanadianencyclopedia.ca/en/article/the-discovery-of-insulin/
[11] https://www.activistfacts.com/organizations/21-people-for-the-ethical-treatment-of-animals/
[11] http://www.understandinganimalresearch.org.uk/why/human-health/polio-vaccine/
[12] https://www.faseb.org/Portals/2/PDFs/opa/Chimpanzees%20in%20Biomedical%20Research_IOM.pdf
[13] https://blog.23andme.com/23andme-and-you/genetics-101/genetic-similarities-of-mice-and-men/
[14] http://fashiondelemmas.weebly.com/blog/animal-testing-pros-and-cons
[16] https://www.peta.org/features/dave-navarro-cruelty-free/
[18] http://www.bbc.co.uk/ethics/animals/using/experiments_1.shtml
[19] https://www.wired.com/2005/07/vioxx-suit-faults-animal-tests/
[20] http://www.neavs.org/research/limitations
[21] https://fbresearch.org/dr-thomas-starzl-transplant-pioneer-animal-research-advocate/