In case a brain tumor is suspected, several tests can be done in order to determine the presence or absence of a brain tumor. The tests can also show what type of tumor it is and can be used to monitor the development of the tumor (growth, spread and other changes) in follow-up examinations. The different options for diagnosing a brain tumor are neurological examinations, brain scans with different imaging modalities and laboratory tests.[1]
Neurological Examinations
If a patient shows symptoms typical to brain tumors, the first step is often a neurological examination. Neurological examinations include a series of tests to analyze a person’s mental state, nerves, senses, muscle strength and reflexes.
Typically, the motor control is tested, as well as abstract thinking and memory.
In detail the following tests are usually made:
- Eye test: vision, movement, pupil reaction
- Hearing test
- Reflex test: patellar reflex
- Balance and coordination test
- Touching test: describing how objects feel
- Smell test: describing various odors
- Facial muscle test: smiling, frowning
- Tongue movement and gag reflex test: swallow response
- Head movement test
- Mental status test: answering easy questions
- Abstract thinking test: interpreting a proverb
- Memory test
In general all effects a brain tumor can show are tested. For example, with the eye test the doctor tests if a tumor exerts pressure on optic nerves. The causes for those effects are described in the article Symptoms of Brain Tumors.
If the clinician suspects a brain tumor after the neurological examination, a brain scan might follow. [1][2]
Brain Scans
Scanning the brain shows clearly if a brain tumor is present and also the position of the tumor. Earlier only anatomical imaging was used, but functional imaging is being incorporated more and more often. Anatomical imaging such as MRI and CT shows the anatomy. Functional imaging such as PET and SPECT uses tracers to track certain functions e.g. increased metabolism due to tumors. Anatomical imaging often has a higher resolution, while functional imaging has a much higher sensitivity. So both methods can be combined in order to determine precise positions of e.g. lesions (PET-CT, PET-MRI). [1][2]
Contrast Agents
Contrast agents (also contrast media or dies) are substances that increase the contrast of certain structures or fluids. They can be injected before imaging. For CT usually very dense materials with high atomic number are used e.g. iodine and barium. For MRI para- or ferro-magnetic substances are used to modify the relaxation times.
Computed Tomography (CT)
Basic principle
The basic principle of Computed Tomography is taking several 2D X-ray images (images of attenuation of rays going through the body, photon energy e = 50-150 keV), which are then reconstructed to a 3D model. The attenuation of X-rays going through the body is tissue dependent - the higher the density the higher the attenuation. So measuring the intensity of X-ray radiation leaving the body gives an insight into the body.
figure: Radiological Imaging. How the intensity is affected by different tissue types [4]
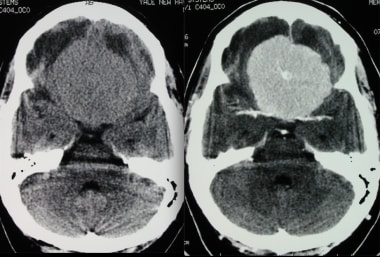
figure: Transverse CT without (left) and with contrast (right) showing brain meningioma. [5]
Contrast agents can be used to enhance the contrast and highlight regions of interest.
What does it show? When is it used?
CT is optimal for bone detection as it shows a high contrast between bones and soft tissue. However it is not easy to distinguish different types of soft tissue, as their attenuation coefficient is in the same range.
A full-body CT scan can be done in few seconds. So in an emergency case often CT is the imaging modality of choice. [3][6]
Magnetic Resonance Imaging (MRI)
Basic principle
The method used for Magnetic Resonance Imaging is nuclear magnetic resonance (this is also the reason why it was formerly known as NMR). The basic concept is the "alignment" of spins in a strong, static magnetic field (1-10 Tesla). Then the thermal equilibrium is disturbed by a radiofrequency irradiation. As every system tends to return to the lowest energy state, it relaxes to the thermal equilibrium and this relaxation is observed. The relaxation times are tissue dependent and so different tissue types show different image contrast.
For this method, nuclei with a total spin of I≠0 are necessary. As more than half of the human body is water, during a MRI H-nuclei (Hydrogen) make up most of the contrast.
T1 and T2 are the longitudinal and the transversal relaxation times. Images can be T1-,T2- or proton density-weighted to visualize different aspects (see figure below).
figure: Soft tissue contrast in MRI. T1-weighted (left upper corner), T2-weighted (right upper), proton density-weighted (left lower), linear combination of T1- and T2-weighted (right lower)
Several 2D images are taken and reconstructed to a 3D model. Contrast agents can be used to achieve further contrast and highlight regions of interest. [3][6][7][8][9][10]
What does it show? When is it used?
MRI is one of the most common modalities used for detecting brain tumors. The strengths of MRI lie in visualizing different types of soft tissue. Therefore it is optimal for brain tumor diagnosis and treatment. Additionally it is not harmful, as it doesn't use ionizing radiation. [3][6][7][8][9][10]
Functional MRI (fMRI)
Functional MRI is magnetic resonance imaging with increased speed. It visualizes how oxygen is used in the brain. It can be used for showing areas with higher neural activity, that means either areas of important functions (to be avoided during the procedure) or areas with abnormally high metabolism (e.g. tumors). [1]
In detail: The blood in the brain is used as "contrast agent". Oxyhemoglobin is diamagnetic, while deoxyhemoglobin is paramagnetic. The metabolism of a brain tumor induces changes: with the neural activity, the blood flow is increasing, which leads to an abnormally high concentration of oxyhemoglobin in certain areas. In these areas the effective relaxation time T2* is increased, leading to a higher contrast to the surrounding tissue. [3]
Flow-Sensitive MRI (FS MRI)
Flow-sensitive or flow-weighted MRI combines images of CSF flow (cerebrospinal fluid) with fMRI. It uses that regional cerebral blood flow is proportional to the difference in the magnetization. [1]
Magnetoencephalography (MEG)
Magnetoencephalography measures the magnetic fields created by nerve cells. It is often combined with other imaging modalities. [1]
Angiography and MRA
Angiography is used to visualize blood vessels in the brain. MRI Angiography (MRA) shows contrast in vessels due to the blood flow using MRI. It can be done with or without contrast agent. [1]
Positron Emission Tomography (PET)
Basic principle
The basic principle of Positron Emission Tomography is the detection of a double gamma emission process after a positron-electron annihilation process. Therefore a positron emitter (β+ decaying substance) is injected. This positron then annihilates with an electron within the body (typically 3-5mm from the β+ emission site), which results in the emission of two photons (511 keV each) in opposite directions. With a circular detector, the line of the event can then be detected. By measuring a lot of those event-lines, the exact location of the annihilation process can be calculated.
figure: Main idea of PET. Annihilation process and line of event [11]
To detect a tumor e.g. radioactivity-marked glucose is injected (e.g. Fluorodeoxyglucose marked with Fluorine-18), which then travels to areas of high metabolism (such as tumors). As shown in the figure below, the brain activity can also be monitored with PET.
figure: PET for assessing brain activity (with F-18-fluorodeoxyglucose (F18-FDG))
What does it show? When is it used?
Positron Emission Tomography is not frequently used for diagnosis, but rather for planning treatment. In can be used to tell the difference between recurrent tumor cells, cells killed by radiation, scar tissue and healthy tissue. It is very sensitive, therefore it can detect even very small lesions. [1][12][13]
Single Photon Emission Computerized Tomography (SPECT)
Basic principle
The basic principle of Single Photon Emission Computerized Tomography is the detection of a single gamma emission decay (γ emitter). Therefore a pure gamma emitter is injected and its decay directly measured to determine the position of the radioactive isotope.
Similar as in PET, to detect a tumor, a radioactive tracer like Tc-99 is injected, which then travels to areas of increased metabolism (e.g. tumors). [12][13]
What does it show? When is it used?
Single Photon Emission Computerized Tomography is also not usually used for diagnosis, but rather for planning treatment. It is similarly sensitive as PET, but a little less accurate in localization. [1][12][13]
Laboratory Tests
For laboratory tests either the indirect potential impact of a tumor is analyzed or a sample of the tumor is resected and examined by a neuropathologist to look for particular cell patterns that are characteristic for different types and grades of brain tumors. Some of the laboratory tests to detect and/or classify tumors are listed below.
Spinal tap/Lumbar puncture
Samples of cerebrospinal fluid (CSF) are taken by inserting a needle into the spinal canal. The needle is inserted usually between the 3rd and 4th lumbar vertebrae. The CSF is examined for substances that indicate the presence of a tumor and analyzed in order to determine if infection, protein, or blood is present in CSF. Analysis may also indicate diseases affecting the central nervous system.
The presence of tumor cells in the CSF after a surgery indicates that the tumor has spread. [1]
figure: Spinal tap. Collecting CSF [14]
Biomarkers
Tiny bits of genetic material (e.g. proteins) from brain tumor cells can be detected in bodily fluids, such as blood, plasma, CSF, urine, and saliva. It may indicate a brain tumor diagnosis, but may also be used for monitoring the effectiveness of medications. In follow-up examinations the doctor especially looks for changes in certain genes in the tumor cells. [1][2]
Biopsy
A biopsy is the removal of a small sample of the brain tumor to aid diagnosis of the tumor type. This operation may take several hours and can only be done if the tumor is accessible. A biopsy can also be part of neurosurgery to resect the whole tumor. [2]
figure: Needle biopsy. Taking a sample of the tumor [15]
The common types of biopsy performed in patients with brain tumors are needle biopsy, stereotactic biopsy and open biopsy.
Like the name says, needle biopsy (picture above) is the removal of tissue with a needle. A cut is made, a small hole drilled into the skull, then a narrow, hollow needle is inserted.
Stereotactic biopsy is a stereotactically guided needle biopsy. A computer-aided guidance system provides the precise location of the tumor and the position of the main structures in the brain (areas of important functions to be avoided).
Open biopsy is done during the resection of the tumor. Therefore it is usually not used for diagnosis. [1]
Evoked potentials
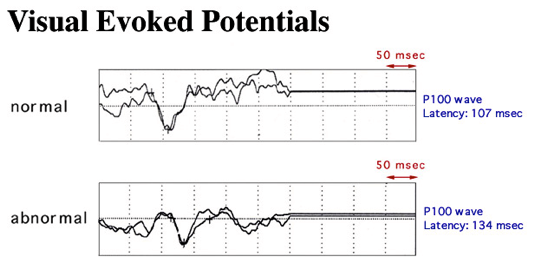
Electroencephalography (EEG) is used to measure the responses to sensory stimulations (e.g. visual, acoustic..). Electrodes are placed onto the patient's head and the patient for example looks at alternating checkerboard pattern. The neural reaction is then analysed. [1][16]
figure: Visual evoked potential. Comparison normal and abnormal [16]
Bibliography
1) Brain Tumor Diagnosis (inter alia), http://www.abta.org/brain-tumor-information/diagnosis/ (access 15/05/17)
2) Getting a diagnosis (inter alia), https://www.thebraintumourcharity.org/understanding-brain-tumours/ (access 15/05/17)
3) Schlegel W. and Bille J. (Eds) (2002) Medizinische Physik – Band 2, Springer: Berlin Heidelberg
4) Johns H.E. & Cunningham J.R. (1980) The Physics of Radiology. 3rd editon,Charles C. Thomas: Springfield, IL.
5) Brain Meningioma Imaging, http://emedicine.medscape.com/article/341624-overview (access 15/05/17)
6) CT and MRI of brain tumors, Bruzzone MG, D'Incerti L, Farina LL, Cuccarini V, Finocchiaro G., https://www.ncbi.nlm.nih.gov/pubmed/22617235
7) Schlegel W. and Mahr A. (Eds) (2007) 3D Conformal Radiation Therapy. A multimedia introduction to methods and techniques, Springer: Heidelberg
8) Oppelt A. (Ed) (2005) Imaging Systems for Medical Diagnostics, Publicis Corporate Publishing: Erlangen
9) Morneburg H. (Ed) (1995) Bildgebende Systeme für die medizinische Diagnostik, Publicis MCD Verlag: Erlangen
10) Dössel O. (2000) Bildgebende Verfahren in der Medizin, Springer: Berlin Heidelberg
11) Lecture Biomedical Physics I, A.4.3. PET- Positron Emission Tomography, Prof. Franz Pfeiffer
12) Phelps M.E. (2006) PET Physics, Instrumentation, and Scanners, Springer, ISBN: 978-0-387-32302-2
13) Wernick M.N. (2004) Emission Tomography: The Fundamentals of PET and SPECT, Academic Press, ISBN: 978-0127444826
14) Cerebral spinal fluid (CSF) collection, http://keckmedicine.adam.com/content.aspx?productId=117&pid=1&gid=003428 (access 15/05/17)
15) Brain tumors: an introduction, http://www.mayfieldclinic.com/PE-BrainTumor.htm (access 15/05/17)
16) Visual Evoked Potential, http://oogziekenhuis.me/Visual_Evoked_Potential/Visual_Evoked_Potential.html (access 15/05/17)