According to cancer.net, the main areas of research of brain tumour diagnosis and treatment, which are at both early and more developed stages are: [1]
- Oncolytic Virus Therapy
- Blood-Brain Barrier disruption
- New drugs and combination of existing drugs
- Immunotherapy
- Palliative care
- Enhanced imaging techniques.
- Biomarkers
From these techniques, in this page we will focus on the first three ones. Enhanced imaging techniques and biomarkers are mostly focused on diagnosis and follow up of the cancer.
These previous treatments were all genetically engineered oncolytic viruses. Along with the success of these techniques, the idea of using naturally occurring viruses was revived. A naturally occurring virus is typically a virus that is not pathogenic in humans.
An oncolytic virus that is currently under research is reolosyn. Reolosyn is a reovirus, i.e a double-stranded RNA virus that replicates preferentially in transformed cells lines but not in normal cells. To learn more about reovirus, please click here.
A phase I trial was conducted in patiens with advance solid tumors. At first some common treatment-related adverse event occurred, like nausea, vomiting, erythema at the injection site, fever and flu symptoms. However, futher studies in phase I demonstrated safety and broad anticancer activity of Reolysin in several cancer types, including malignant glioma and multiple myeloma. In phase II, it was studied via intravenous and intralesional administration. Phase III trials were performed comparing the combination of Reolysin with some chemotherapy drugs, and the chemotherapy alone.
Finally, the FDA granted Reolysin an orphan drug designation for malignant glioma in 2015, among other. An orphan drug is a drug defined intended for the safe and effective treatment, diagnosis or prevention of rare diseases or disorders that affect fewer than 200.000 people in the US, or affect more persons but are not expected to revocer the costs of developing and marketing a treatment drug [4] .
New Drugs and Combination of Existing Drugs
There is been research about using drugs for other types of cancer as treatment for brain tumour. Moreover, combination of different drugs that attack different paths of the tumour growth and spread. Also the tumours may develop resistance to the drugs administrated, therefore there’s research about attacking the tumour cells resistance capacity.
Researches at Sanford Burnham Prebys Medical Discovery Institute [8] have identified a new combination therapy for the most aggressive form of medulloblastoma, a pediatric brain cancer with only 40% survival rate. The combination consists on two drugs, histone deacetylase inhibitors (HDACIs) and phosphatidylinositol 3-kinase inhibitors (PI3KIs), and it has been shown to kill both mouse and human medulloblastoma cells with minimal toxicity. Both drugs have been revealed as promoters of the FOXO1 activity, a gene that interferes with the MYC oncogene. The combination of both significantly improved survival mice with human MYC tumours compared to each drug on its own. This research was published in March 2016, and it's been on clinical trials to confirm its benefits.
Furthermore, one of the main concerns about chemotherapy is the possible toxicity of the anticancer drugs administrated to the patient. The ideal scenario is to develop drugs that only attack cancer cells, while not affecting healthy cells and minimizing the side effects. This issue has been addressed via targeted therapy [9]. These treatment consists on anticancer drugs with reduced toxicity and targeted delivery methods. This strategy is based on specificity or high selectivity of binding to tumour cells. This can occur exploiting over-expression of receptors by tumour cells or expression of receptors not found on normal brain cells. Several pathways are involved in the oncogenic process in gliomas. Therefore, novel targeted therapies are based on the use of inhibitors of the factors produced on these pathways. If you want to know more about targeted therapy and examples of this treatment, please click here.
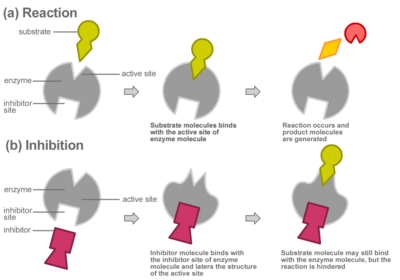
From left to right. [10] Action mechanism of an inhibitor, which binds to the active site of the enzyme, preventing the binding of the substrate and thus no reaction occurs. [11] Development process from astrocyte to primary gliobastoma. These changes are genetic and come with an up- or down-regulation of certain factors.
Immunotherapy
According to cancer.org [12] immunotherapy is a type of treatment that uses certain parts of a person's immune system to fight diseases such as cancer. This can be done in two different ways:
- Stimulating the immune system to work harder so it attacks the cancer cells.
- Give external immune system components, such as man-made immune system proteins.
This treatment method was grown during the last few decades. It can either boost the own immune system of trains it to specifically attack the cancer cells. There are different types of cancer immunotherapy:
- Monoclonal antibodies: artificial versions of immune system proteins. They can be design to attack an specific part of a cancer cell.
- Immune checkpoint inhibitors: These drugs help the immune system to recognise and attack the cancer cells.
- Cancer vaccines
- Other, non-specific: They boost the immune system in a general way.
The areas where most research it's been focused on and more promising are monoclonal antibodies, cancer vaccines and combination between immunotherapy and targeted therapy.
Monoclonal antibodies (mAbs)
Researches are building these mAbs to exploit the differences between cancer and healthy cells. Moreover, they can also be attached to drugs and other substances in order to make them more powerful.
However, as mAbs are proteins, the own immune system can react against them. This can lead to side effects as well as destroying the mAbs. Therefore, new forms of mAbs are been discussed to reduce the immune reactions. Furthermore, another approach is the combination of two antibodies (bispecific antibody). One part attaches to a cancer cell while the other attaches to an immune cell. Both cells are brought together leading to an immune response.
In brain tumour treatment, the target is selected so for example it is expressed by the brain tumour but not normal in the central nervous system tissue. One example is Tenascin, an extracellular protein expressed in fibrillary matrix and the perivascular space of various tumours, including gliomas, but not in the normal brain. Several tenascin specific mAbs are in clinical trials in the US and Europe. Also several growth factors are been studied as target candidates.
Cancer Vaccines
This treatment is not yet a major type of cancer treatment. Due to the complexity of the immune system and the capacity of cancer cells to elude the immune system, the development is still on early stages. There are several types of cancer vaccines:
- Tumour cell vaccines: They are made of actual cancer cells that have been removed from the patient during surgery. The cells are altered in the lab to make them more likely to the attacked by the immune system and then introduced back to the patient. The immune system thus will attack these cells and any similar ones in the body. The cancer cells can come from the patient himself (autologous) or from other patient (allogenic). The last one are easier to make but still unknown if they are more effective than the first ones.
- Antigen vaccines: They boost the immune system by using one or few antigen, usually proteins or pieces of proteins (peptides). They can be specific for a type o cancer but not patient specific.
- Dendritic cell vaccines: This kind of vaccines have shown the most success in treating cancer. These cells help the immune system to recognise cancer cells. They break them down into antigens and hold them so other immune cells called T cells can see them. The T cells start an immune reaction against any cells in the body that contain these antigens. Dendritic cell vaccines are made from the person in whom they will be used. The process is complex and expensive.
- Vector-based vaccines: These vaccines use special delivery systems called vectors to make them more efficient. Vectors are usually viruses, bacteria and yeast cells. These vaccines are easier and less expensive. Moreover, as the vector itself can trigger their own immune system responses from the body, they could help make the overall immune system response stronger. Also they can deliver more than one cancer antigen at a time, which can make the body's immune response more likely to react. This method is specially used for treating gliobastomas.
Researches from the City of Hope Beckman Research Institute, led by Dr. Behnam Badie, are undergoing a clinical trial with nine gliobastoma patients, where some immune cells are extracted from the patients, modified in the lab and injected back. These patients had already undergone surgery, radiation and drug therapies but the cancer had returned and spread to other parts of the brain. The outcome of this treatment is a significant decrease of growth is several parts of the tumour.
These promising results encourage and strengthen the idea of immunotherapy to control cancer. If you want to know more about this research, please click here
Immune checkpoint inhibitors
The immune system has checkpoints proteins that avoid it from attacking normal cells in the body. Cancer cells sometimes take advantage of these checkpoints to avoid being attacked by the immune system. Research is focused then in targeting these checkpoints as this treatment could be used for different types of cancer. However only a few of these treatments have been approved and many others are being studied in clinical trials. Clinical areas for melanoma and some types of lung cancer have seen success regarding this treatment, while the efficacy in brain tumour (gliobastoma) is still undetermined.
Bibliography
[1] http://www.cancer.net/cancer-types/brain-tumor/latest-research
[2] http://onlinelibrary.wiley.com/doi/10.1111/cas.13027/full
[3] http://islaslab.blogspot.de/2014/05/oncolytic-viruses-homing-missiles-of.html
[4] https://www.fda.gov/forindustry/DevelopingProduCTsforrareDiseasesConditions/default.htm
[6] D.Kobiler, S.Lustig, S.Shapira, 2012, Blood-Brain Barrier: Drug Delivery and Brain Pathology. Pages 259-263.
[7] http://fennecpharma.com/wp-content/uploads/2013/06/Neuwelt-E-A-et-al-1998-Paper.pdf
[8] V.Laquintana, A.Trapani, N.Denora, 2009, New Strategies to Deliver Anticancer Drugs to Brain Tumors. Source: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2752842/
[9] https://medicalxpress.com/news/2016-03-drug-combination-childhood-brain-cancer.html#jCp
[10] http://www.nature.com/nrd/journal/v3/n6/fig_tab/nrd1414_F1.html
[11] http://www.newworldencyclopedia.org/entry/Enzyme
[14] C.Wikstrand, M.Zalutsky, 2004, Monoclonal antibodies for brain tumour treatment.
[15] http://time.com/4618566/brain-cancer-treatment-immunotherapy/
[16] A.Luksik, R.Maxwell, 2017, The Role of Immune Checkpoint Inhibition in the Treatment of Brain Tumours. Source: https://www.ncbi.nlm.nih.gov/pubmed/28258545